The US Food and Drug Administration (FDA) has authorized the use of Leqembi, a drug designed to slow the progression of Alzheimer’s disease. It is noted that this is the only drug of its kind approved by the FDA.
Leqembi uses antibodies that target amyloid beta. This protein plays an important role in the development of Alzheimer’s disease.
People with this disease have an abnormally formed form of amyloid. Over time, it accumulates in the brain, causing the development of strong clots called plaques. These plaques, along with the accumulation of another misfolded protein called tau, contribute to the gradual destruction of the brain.
Breaking up or preventing plaque formation can stop or slow down the decline in people’s cognitive abilities.
The effectiveness of the drug in slowing the development of Alzheimer’s disease
In a pivotal 18-month clinical trial, the drug was found to slow the progression of cognitive decline by 27% in patients compared to those taking placebo. The subjects also performed better on tests of daily functioning and had lower levels of amyloid in their brains.
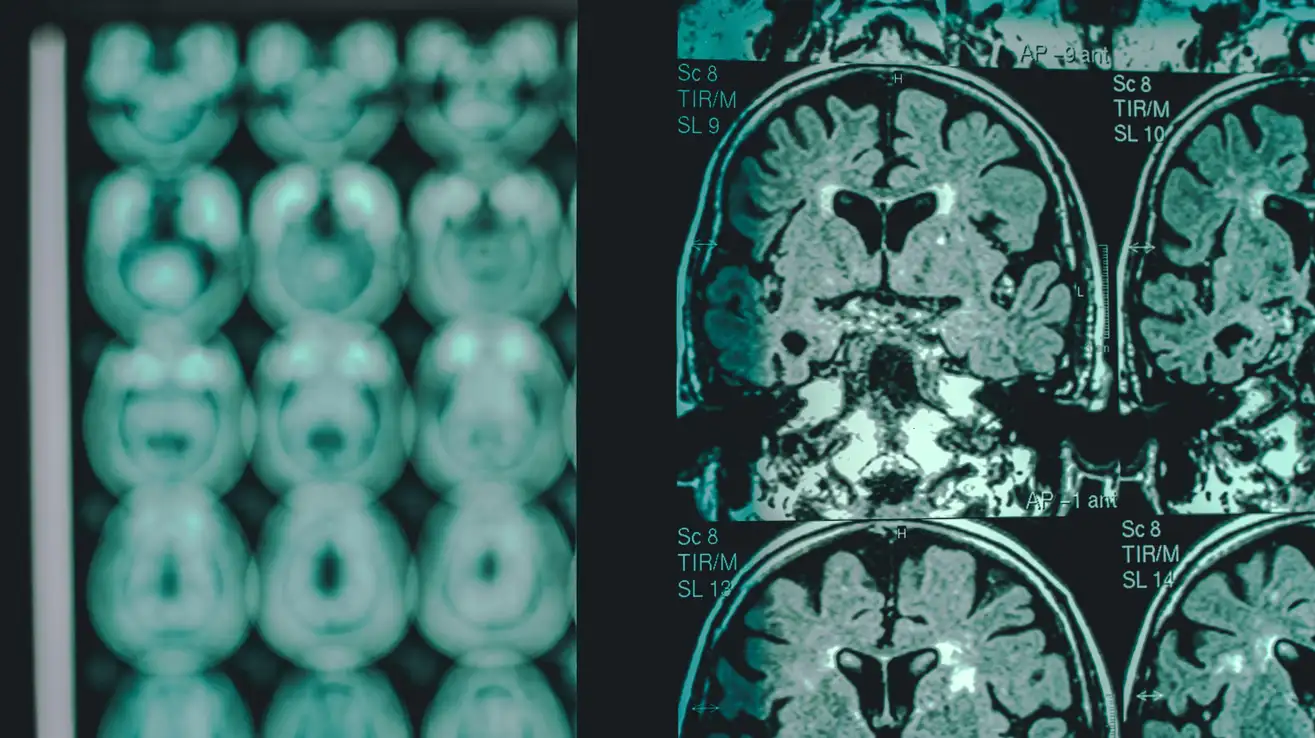
However, the drug has side effects. One of the most common complications is known as amyloid imaging abnormalities (ARIA). They can be diagnosed only with MRI.
ARIAs are usually caused by temporary swelling of the brain, but sometimes they can be a sign of a dangerous hemorrhage. Most of the anomalies are not life-threatening, but several fatalities have been reported.
The risk of ARIA is higher in people who have an ApoE ε4 mutation associated with Alzheimer’s disease. The use of blood thinners may also be another risk factor for cerebral bleeding in these patients.

